Beautiful Info About What Is The PE Of Two Charges

Basic Properties Of Electric Charge Study Material For IIT JEE
Unveiling the Potential
1. Understanding the Basics
Ever wondered why some things just seem to "want" to move? Like a ball rolling downhill, or two magnets snapping together? Well, a lot of that has to do with something called potential energy. Specifically, when we're talking about electricity, it's the electrical potential energy (PE) that gets the show on the road. And when we've got two electrically charged objects hanging around each other, things get interesting...and potentially energetic!
Think of it like this: imagine holding a spring compressed. You're putting energy into that spring by forcing it into a state it doesn't naturally want to be in. That compressed spring has potential energy the potential to do something, like launch a small projectile across the room. Electrical potential energy is similar, but instead of springs, we're dealing with the forces between electric charges.
So, what exactly is the electrical potential energy of two charges? In its simplest form, it's the amount of work required to bring those two charges from an infinite distance apart to their current positions. "Infinite distance," you say? Yeah, it's a bit of a theoretical starting point, but it helps us define a nice zero point for our energy calculations. Basically, the closer two charges are (and depending on their signs - more on that later!), the higher or lower their potential energy.
The key takeaway here is that potential energy tells us how much "oomph" is stored in the configuration of those two charges. This "oomph" can then be converted into kinetic energy — the energy of motion — if the charges are allowed to move freely. Think of letting go of that compressed spring: all that potential energy gets converted into the projectile's movement.

Electric Potential And Capacitance Ppt Download
The Math Behind the Magic
2. Diving into the Formula
Okay, now for the slightly more technical bit (don't worry, it's not that scary). The electrical potential energy (U) of two point charges is given by the following formula:
U = k q1 q2 / r
Where:
- U is the electrical potential energy (measured in Joules)
- k is Coulomb's constant (approximately 8.99 x 10^9 Nm^2/C^2)
- q1 and q2 are the magnitudes of the two charges (measured in Coulombs)
- r is the distance between the two charges (measured in meters)
Let's break this down. `k` is just a constant, so we don't need to worry about it too much. The important part is the `q1 q2 / r`. This tells us that the potential energy is directly proportional to the product of the charges and inversely proportional to the distance between them.
This formula reveals a few interesting things. First, if both charges are positive or both are negative, their product will be positive, leading to a positive potential energy. This means you need to put in energy to bring those charges together, because they naturally repel each other. It's like pushing those similar poles of magnets together - you have to work against the repelling force. Conversely, if one charge is positive and the other is negative, their product will be negative, resulting in a negative potential energy. This means the charges are attracted to each other and they release energy as they get closer. They're happy to be together! It's like the north and south poles of magnets attracting.
Positive vs. Negative Potential Energy: It Matters!
3. Attraction and Repulsion
This is where it gets a bit philosophical (okay, maybe not philosophical , but definitely important). The sign of the potential energy whether it's positive or negative tells us a lot about the interaction between the two charges. A positive potential energy means the charges are repelling each other. They're in a state of tension, like that compressed spring we talked about earlier. You had to do work to bring them together, and they're just itching to get away from each other.
On the other hand, a negative potential energy means the charges are attracting each other. They're in a state of harmony, like two puzzle pieces fitting together perfectly. They release energy as they move closer because they're moving in the direction of the force acting between them. Think of it like letting a ball roll downhill — gravity is doing the work, and the ball is gaining kinetic energy as it loses potential energy.
So, when you see a positive potential energy, think "repulsion." When you see a negative potential energy, think "attraction." This simple association will help you understand the dynamics of charged particles much better. It also impacts the work required to separate charges and to bring them together.
Ignoring the sign is like ignoring half the story! Make sure to pay attention to whether the potential energy is positive or negative because it completely flips the script on what the charges are "trying" to do.
Beyond Two Charges: What About More?
4. Adding More to the Mix
Alright, we've mastered the art of potential energy with two charges. But what happens when we throw a third charge into the mix? Well, things get a little more complicated, but the underlying principle remains the same. The total potential energy of a system of multiple charges is simply the sum of the potential energies of all the possible pairs of charges.
For example, if we have three charges (q1, q2, and q3), we need to calculate the potential energy between q1 and q2, between q1 and q3, and between q2 and q3. Then, we add those three potential energies together to get the total potential energy of the system. This is based on the principle of superposition the total effect is the sum of individual effects.
Mathematically, it looks like this:
U_total = U_12 + U_13 + U_23
Where U_12 is the potential energy between q1 and q2, U_13 is the potential energy between q1 and q3, and U_23 is the potential energy between q2 and q3.
As you add even more charges, the number of pairs increases rapidly, and the calculation becomes more tedious. But the concept remains the same: calculate the potential energy for each pair and then add them all up. This is why computers are often used for these calculations when dealing with a large number of charges.
Practical Applications: Where Does This Stuff Show Up?
5. Real-World Relevance
Okay, so all this talk about potential energy and charges might seem a bit abstract. But it actually has tons of practical applications in the real world! Understanding the potential energy between charges is crucial in fields like chemistry, materials science, and electronics.
For example, chemical bonds are essentially the result of the electrostatic attraction between positively charged nuclei and negatively charged electrons. The potential energy between these charged particles determines the strength and stability of the chemical bond. This is why understanding electrostatic potential is so vital in modeling chemical reactions and designing new molecules.
In electronics, the behavior of transistors and other semiconductor devices depends critically on the electric fields and potential energies created by the distribution of charges within the material. Designing efficient and reliable electronic devices requires a deep understanding of these concepts. Ever wondered how your phone works? Yeah, potential energy calculations are part of the equation!
Even in larger scale applications, such as designing capacitors (devices that store electrical energy), understanding the relationship between charge, voltage, and potential energy is absolutely essential. So, while it might seem like a theoretical concept, electrical potential energy is a fundamental tool in a wide range of scientific and technological fields.
FAQ: Potential Energy of Two Charges - Quick Questions Answered
6. Q: What happens to the potential energy if I double the distance between two charges?
A: If you double the distance between two charges, the potential energy is halved (assuming all other factors remain constant). This is because potential energy is inversely proportional to the distance (r) between the charges, as shown in the formula U = k q1 q2 / r.
7. Q: Can potential energy be zero?
A: Yes, potential energy can be zero. This usually happens when the charges are infinitely far apart (which is a theoretical starting point), or if either of the charges is zero. The zero point for potential energy is arbitrary; it's the change* in potential energy that's physically meaningful.
8. Q
A: Potential energy is a scalar quantity. It has magnitude but no direction. The force between the charges is a vector, but the potential energy is simply a value that represents the stored energy due to the configuration of the charges.

How Electrostatic Potential Due To A Point Charge Is Vrogue.co
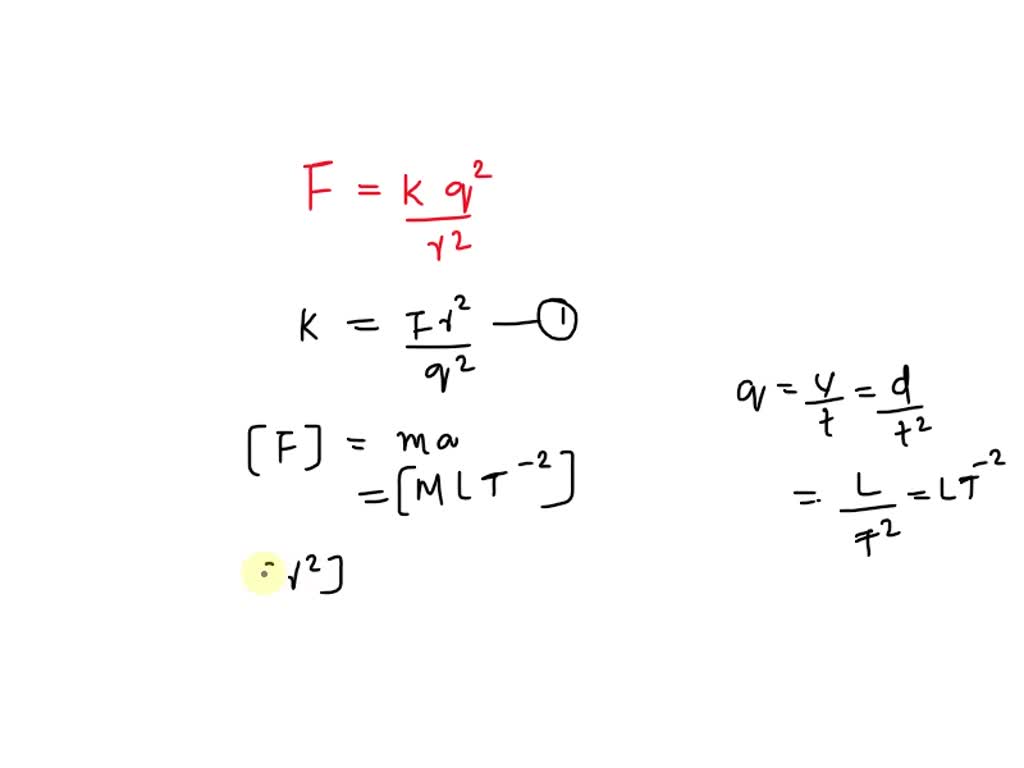

Electrostatic Potential Energy Of A System Charges On Equilateral
